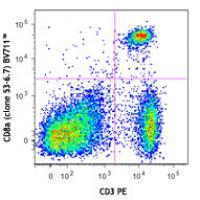
| Description | CD8, also known as Lyt-2, Ly-2, or T8, consists of disulfide-linked α and β chains that form the α(CD8a)/β(CD8b) heterodimer and α/α homodimer. CD8a is a 34 kD protein that belongs to the immunoglobulin family. The CD8 α/β heterodimer is expressed on the surface of most thymocytes and a subset of mature TCR α/β T cells. CD8 expression on mature T cells is non-overlapping with CD4. The CD8 α/α homodimer is expressed on a subset of γ/δ TCR-bearing T cells, NK cells, intestinal intraepithelial lymphocytes, and lymphoid dendritic cells. CD8 is an antigen co-receptor on T cells that interacts with MHC class I on antigen-presenting cells or epithelial cells. CD8 promotes T cell activation through its association with the TCR complex and protein tyrosine kinase lck. |
| Recommended Usage | Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For immunofluorescent staining using the microg size, the suggested use of this reagent is ≤0.5 microg per million cells in 100 microL volume. For immunofluorescent staining using microL sizes, the suggested use of this reagent is ≤5 microL per million cells or 5 microL per 100 microL of whole blood. It is recommended that the reagent be titrated for optimal performance for each application.
Brilliant Violet 711™ excites at 405 nm and emits at 711 nm. The bandpass filter 710/50 nm is recommended for detection, although filter optimization may be required depending on other fluorophores used. Be sure to verify that your cytometer configuration and software setup are appropriate for detecting this channel. Refer to your instrument manual or manufacturer for support. Brilliant Violet 711™ is a trademark of Sirigen Group Ltd.
This product is subject to proprietary rights of Sirigen Inc. and is made and sold under license from Sirigen Inc. The purchase of this product conveys to the buyer a non-transferable right to use the purchased product for research purposes only. This product may not be resold or incorporated in any manner into another product for resale. Any use for therapeutics or diagnostics is strictly prohibited. This product is covered by U.S. Patent(s), pending patent applications and foreign equivalents. |
| References | 1. Ledbetter JA, et al. 1979. Immunol. Rev. 47:63. (IHC, IP)
2. Hathcock KS. 1991. Current Protocols in Immunology. 3.4.1. (Deplete)
3. Takahashi K, et al. 1992. P. Natl. Acad. Sci. USA 89:5557. (Block, IP)
4. Ledbetter JA, et al. 1981. J. Exp. Med. 153:1503. (Block)
5. Hata H, et al. 2004. J. Clin. Invest. 114:582. (IHC)
6. Fan WY, et al. 2001. Exp. Biol. Med. 226:1045. (IHC)
7. Shih FF, et al. 2006. J. Immunol. 176:3438. (FC)
8. Kamimura D, et al. 2006. J. Immunol. 177:306.
9. Bouwer HGA, et al. 2006. P. Natl. Acad. Sci. USA 103:5102. (FC, Deplete)
10. Kao C, et al. 2005. Int. Immunol. 17:1607. PubMed
11. Ko SY, et al. 2005. J. Immunol. 175:3309. (FC) PubMed
12. Rasmussen JW, et al. 2006. Infect. Immun. 74:6590. PubMed
13. Lee CH, et al. 2009. Clin. Cancer Res. PubMed
14. Geiben-Lynn R, et al. 2008. Blood 112:4585. (Deplete) PubMed
15. Kingeter LM, et al. 2008. J. Immunol. 181:6244. PubMed
16. Guo Y, et al. 2008. Blood 112:480. PubMed
17. Andrews DM, et al. 2008. J. Virol. 82:4931. PubMed
18. Britschqui MR, et al. 2008. J. Immunol. 181:7681. PubMed
19. Kenna TJ, et al. 2008. Blood 111:2091. PubMed
20. Jordan JM, et al. 2008. Infect. Immun. 76:3717. PubMed
21. Todd DJ, et al. 2009. J. Exp. Med. 206:2151. PubMed
22. Bankoti J, et al. 2010. Toxicol. Sci. 115:422. (FC) PubMed
23. Medyouf H, et al. 2010. Blood 115:1175. PubMed
24. Riedl P, et al. 2009. J. Immunol. 183:370. PubMed
25. Apte SH, et al. 2010. J. Immunol. 185:998. PubMed
26. Bankoti J, et al. 2010. Toxicol. Sci. 115:422. (FC) PubMed
27. del Rio ML, et al. 2011. Transpl. Int. 24:501. (FC) PubMed |